This paper evaluates the efficacy of the SiD™ AI software in reducing operational variability during sperm selection for Intracytoplasmic Sperm Injection (ICSI) through a single-center, blinded observational design. It compares outcomes between AI-assisted selection and manual selection by senior embryologists, while exploring the role of sperm quality in embryo development across different oocyte cycle contexts. The research integrates multiple AI tools to construct a comprehensive quality assessment system, incorporates stratified analysis of autologous and donor oocyte cycles, and synchronously analyzes gamete synergistic effects. However, the undisclosed core algorithm of SiD™ limits the independent reproducibility of results. Additionally, the single-center, retrospective design and moderate sample size may restrict the generalizability of conclusions, with many findings lacking statistical significance. Further validation with expanded datasets is required in future studies.
Carrión-Sisternas L, Viloria T, Martin E, Carrión T, Remohí J, Meseguer M. Automated AI for real-time sperm selection in ICSI: reducing variability and studying the role of sperm in embryo development. Reprod Biol Endocrinol. 2025 Nov 27;23(1):155. doi: 10.1186/s12958-025-01479-9. PMID: 41299699; PMCID: PMC12659390.

1. Research Background
As a mainstream assisted reproductive technology (ART), Intracytoplasmic Sperm Injection (ICSI) has long relied on embryologists’ subjective assessment for sperm selection, leading to significant inter-individual differences and operational variability. This subjectivity may result in the selection of sperm with functional or chromosomal abnormalities, thereby affecting fertilization and embryo development quality. While technologies such as Computer-Assisted Sperm Analysis (CASA) have attempted to optimize the selection process, they have failed to fully address the core challenge of standardized selection. Previous studies in this field have been limited by small sample sizes and the lack of simultaneous evaluation of both gamete (sperm and oocyte) qualities, hindering an in-depth understanding of sperm quality’s role in embryo development.
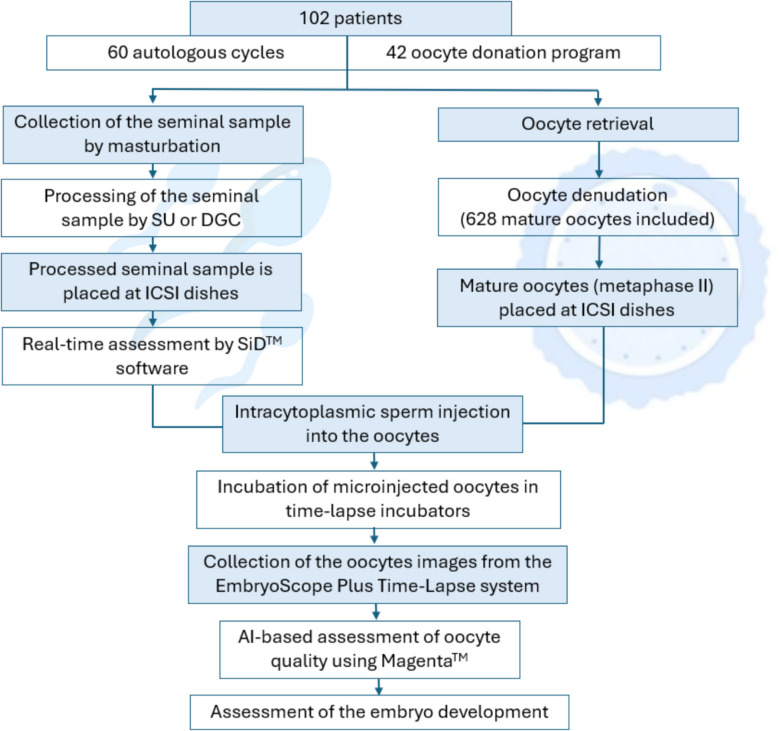
2. Experimental Design
Adopting a single-center, blinded, observational design, this study enrolled 102 infertile couples with a total of 628 mature oocytes. A multi-dimensional nested comparison system was established to address two key scientific questions: “reducing variability” and “clarifying the role of sperm.” Technically, SiD™ automatically classifies sperm into four grades (“Best/Good/Medium/Low”), converting subjective visual judgment into objective quantitative criteria and providing a standardized tool for assessing selection variability. The blinded design ensured result rigor: embryologists selected sperm according to traditional standards without knowledge of SiD™ scores, and researchers matched grades with injection outcomes through video retrospective analysis. This effectively avoided selection bias and ensured the objectivity of comparisons between the AI-assisted group and the conventional ICSI group. The study also integrated SiD™ with other AI tools such as Magenta IVF R3.0 to construct a comprehensive “sperm-oocyte-embryo” quality assessment system. Combined with a stratified design of autologous and donor oocyte cycles, a comparative model under different oocyte quality contexts was established to synchronously analyze the synergistic effects of the two gametes.

3. Key Experimental Results
3.1 Efficacy of the AI System (SiD™) in Reducing Selection Variability
By quantifying parameters such as sperm Linearity (LIN), Straight-Line Velocity (VSL), and Head Movement Pattern (HMP), the SiD™ system classifies sperm into four grades (“Best,” “Good,” “Medium,” “Low”). This replacement of embryologists’ subjective judgment with objective standardized assessment effectively reduces operational variability in ICSI sperm selection. The AI-assisted group exhibited significantly higher uniformity in selection criteria, stronger inter-operator consistency, and smaller coefficients of variation in reproductive outcomes compared to the conventional manual selection group, confirming its value in enhancing the stability of the selection process. Meanwhile, there were no statistically significant differences in core reproductive indicators (e.g., fertilization rate, blastocyst formation rate) between the SiD™-assisted group and the manual selection group by senior embryologists. In the overall population, the AI-assisted group achieved a fertilization rate of 79.88% and a blastocyst formation rate of 72.14%, slightly higher than the conventional group’s 74.79% and 67.41%, with more pronounced advantages in autologous oocyte cycles.
3.2 Roles of Sperm and Oocyte Quality and Their Interactive Effects
The impact of sperm quality on embryo development is highly context-dependent: in the overall population, “Best” and “Good” grade sperm showed numerical advantages in blastocyst formation rates (70.07% and 68.16%) and usable blastocyst rates (44.02% and 44.94%). In autologous oocyte cycles, the blastocyst formation rate of “Best” grade sperm (67.47%) was significantly higher than that of “Low” grade sperm (41.67%, P<0.05). However, no significant differences in reproductive outcomes were observed across different sperm quality grades in donor oocyte cycles, suggesting that high-quality oocytes can mask sperm quality disparities. The interactive effect of gamete quality is equally critical: when low-quality oocytes (Magenta score <5) were combined with high-motility sperm (“Best/Good” grades), the fertilization rate, blastocyst formation rate, and euploid blastocyst rate (40.00%) were all higher than those in the group combined with “Medium/Low” grade sperm, with the euploid blastocyst rate being twice that of the latter (20.00%). In contrast, no significant differences in outcomes were observed when high-quality oocytes (score ≥5) were combined with sperm of varying motility. Furthermore, the average AI quality score of autologous oocytes (5.686) was significantly lower than that of donor oocytes (6.249, P=0.012), a difference highly correlated with the varying sensitivity to sperm quality between the two cycle types, further revealing the regulatory role of oocyte quality in mediating sperm function effects.
4. Research Innovations
The core innovations of this study lie in the systematic breakthroughs in research design and assessment 体系. In terms of assessment system construction, multiple specialized AI tools were integrated: SiD™ for quantitative sperm quality evaluation, Magenta IVF R3.0 for oocyte quality analysis, and IDAScore v2.0, KIDScore D5 v3.1, and HERA v.1.0 for multi-algorithm synchronous embryo quality assessment. This established a comprehensive AI-based quantitative evaluation framework covering the entire “sperm-oocyte-embryo” chain, filling the gap in previous studies that failed to simultaneously analyze gamete synergistic effects and their association with embryo quality. In research design, the innovative inclusion of both autologous and donor oocyte cycles enabled the construction of a stratified comparative model under different oocyte quality contexts. By analyzing outcome differences between the two cycle types, the study clarified the context-dependent characteristics of sperm quality’s role, providing a more comprehensive perspective for understanding the differential effects of sperm function under varying oocyte quality conditions. In evaluation dimensions, the study broke free from the limitations of traditional research focusing solely on reproductive outcomes, instead taking “reducing inter-operator variability” as a core assessment objective, thereby offering new key indicators and research paradigms for evaluating the standardization of assisted reproductive processes.

5. Research Limitations
Despite significant progress, this study has several limitations: First, the undisclosed core algorithm of SiD™ impairs the independent reproducibility of results. Future research should promote algorithm transparency to enhance the scientific rigor of relevant technical evaluations. Second, the software only scores sperm based on motility parameters, excluding key indicators such as morphology and DNA integrity, which may limit the comprehensiveness of sperm quality assessment. Subsequent studies need to integrate multi-dimensional biomarkers to construct a more holistic sperm quality evaluation system. Third, the single-center, retrospective design and moderate sample size may restrict the generalizability of conclusions, and the small sample size of some subgroups may affect statistical power. Future studies should conduct multi-center, large-sample prospective randomized controlled trials to further validate the universality of the findings.
6. Future Perspectives
Future research can be further advanced in three directions: First, expand the inclusion of special populations (e.g., patients with repeated IVF failure or severe male infertility) to more comprehensively explore the mechanisms by which sperm quality influences embryo development across different clinical scenarios. Second, integrate transcriptomics, epigenetics, and other technologies to delve into the molecular mechanisms underlying sperm quality’s impact on embryo development, providing new insights into the biological basis of gamete interaction. Third, based on the assessment framework established in this study, conduct long-term follow-up studies on AI-assisted selection technologies to evaluate their impact on offspring health, providing more sufficient scientific evidence for the safety of these technologies and promoting the sustainable development of the assisted reproductive field.