With the rapid advancement of assisted reproductive technology (ART), embryo quality assessment remains a critical determinant of the success rate of in vitro fertilization-embryo transfer (IVF-ET). For decades, clinicians have primarily relied on the Gardner scoring system based on two-dimensional (2D) microscopic imaging to evaluate blastocyst quality. However, this method inherently suffers from strong subjectivity and the inability to capture the three-dimensional (3D) structure of embryos. Recently, a research team from Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, among other institutions, published a significant study in npj Digital Medicine, introducing a non-invasive, clinically compatible 3D blastocyst reconstruction technology that provides a more objective and accurate new scheme for embryo assessment.

Dilemmas and Challenges of Traditional Embryo Assessment
Blastocyst transfer has become the mainstream strategy in ART due to its high implantation rate, and the accurate evaluation of embryo quality is directly linked to pregnancy outcomes. The widely used Gardner scoring system grades blastocysts based on the morphology of the inner cell mass (ICM), trophectoderm (TE), and degree of expansion. Nevertheless, this manual assessment method has obvious limitations.
Research data shows that the consistency of ICM grading for fully expanded blastocysts among 10 embryologists from different clinics was only moderate (Kappa = 0.349), and the consistency of TE grading was similarly unsatisfactory (Kappa = 0.397). This indicates that different physicians may have significant discrepancies in evaluating the same embryo, making subjective errors unavoidable. More importantly, 2D imaging can only capture partial cross-sectional information of the embryo, and the morphological characteristics of ICM and TE may vary with imaging angles, further affecting assessment accuracy. Although 3D reconstruction technology has been proven to enable more comprehensive evaluation of embryo morphology, existing methods either require fluorescent labeling that causes embryo damage or involve complex operations that disrupt the culture environment, making them difficult to integrate into routine clinical workflows. The development of a non-invasive, efficient, and clinically compatible 3D assessment method has become an urgent need in the field of ART.

Innovative Technology: Non-Invasive 3D Reconstruction
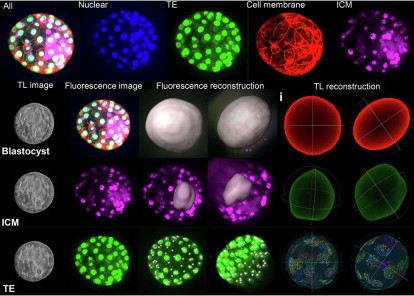
To address the shortcomings of traditional technologies, the research team developed a 3D blastocyst reconstruction method based on a time-lapse (TL) imaging system and artificial intelligence (AI) algorithms. The core workflow of this technology is concise and efficient, fully relying on conventional clinical equipment. The team collected blastocyst data from 2025 frozen-thawed embryo transfer (FET) cycles, with each blastocyst corresponding to imaging data from 11 focal planes. Due to the limited number of original focal planes, the team adopted a generative adversarial network (GAN)-based interpolation algorithm to expand the 11 focal planes to 81, effectively compensating for the insufficient vertical resolution and providing sufficient data support for accurate reconstruction.

In the image segmentation stage, a UNet++ deep learning model was used for high-precision semantic segmentation of ICM and TE in blastocysts. All images were annotated and reviewed by two senior embryologists with more than 15 years of experience in accordance with expert consensus. The team improved the clarity of cell edges through image enhancement technology, ultimately achieving excellent segmentation results—with an accuracy of 0.679 for ICM segmentation in the training set and 0.797 for TE segmentation, respectively. The 3D reconstruction process consists of geometric structure reconstruction and texture feature reconstruction. Point cloud models are constructed by extracting the boundary contours of ICM and TE, followed by surface reconstruction to form grid geometric structures, while integrating texture information from multiple focal planes to generate complete 3D models.
To verify the reconstruction accuracy, the research team selected 26 blastocysts for fluorescent staining and used confocal microscopy reconstruction as the “gold standard” control. The results showed that the relative errors of the core parameters reconstructed by this technology were extremely low: the blastocyst surface area error was only 2.13% ± 1.63%, the diameter error was 1.98% ± 1.32%, and the TE cell count error was 10.00% ± 8.73%, fully demonstrating the reliability of the method.

Key Findings: 3D Parameters Associated with Pregnancy Outcomes
The research team successfully quantified 20 3D morphological parameters of blastocysts, covering four categories: overall blastocyst morphology, TE-related characteristics, ICM-related characteristics, and ICM-TE spatial relationships. They systematically analyzed the associations between these parameters and clinical outcomes such as pregnancy, live birth, and miscarriage.
Data analysis revealed that multiple 3D parameters were significantly correlated with pregnancy and live birth outcomes. Specifically, larger blastocyst surface area, volume, diameter, and blastocoel volume, along with a smaller surface area/volume ratio, were associated with higher probabilities of pregnancy and live birth (P < 0.001). TE-related indicators such as surface area, volume, cell count, and density were also closely linked to pregnancy outcomes, with higher values indicating better embryonic developmental potential (P < 0.001). Among ICM-related parameters, the shape factor emerged as a key predictor of pregnancy outcomes—a smaller shape factor (i.e., a more spherical ICM) was associated with higher success rates of clinical pregnancy and live birth (P < 0.05). Additionally, a larger spatial distance between ICM and TE and a smaller proportion of ICM volume to blastocyst volume were more conducive to successful pregnancy (P < 0.05). In contrast, a larger ICM surface area/volume ratio and a higher number and proportion of TE cells within the ICM quadrant were significantly associated with an increased risk of miscarriage (P < 0.05).
Notably, this technology can effectively distinguish embryos that are difficult to differentiate using the traditional scoring system. Among the 2025 blastocysts included in the study, 4BB-grade blastocysts accounted for as high as 43.7% (885 cases), and traditional methods cannot further distinguish their developmental potential. However, 3D parameter analysis showed that TE surface area and TE volume were significantly correlated with pregnancy, while blastocyst volume and surface area/volume ratio were significantly correlated with live birth (P < 0.05). This indicates that the technology can help clinicians select embryos with greater developmental potential among those with similar grades. The study also found significant differences in the 3D morphology of ICM between female and male embryos (P < 0.05), with female embryos having a relatively larger and more spherical ICM, providing a new perspective for in-depth research on embryonic development mechanisms.

Clinical Value and Future Prospects
This innovative technology brings multiple clinical values to the field of ART. It overcomes the subjective limitations of traditional 2D assessment by providing objective and unified evaluation criteria through quantified 3D parameters, helping to reduce inter-physician variability and improve the consistency and accuracy of embryo selection. Meanwhile, the technology does not require changes to existing clinical workflows and can be directly integrated into routine embryo culture and assessment, allowing medical institutions to promote its application without additional substantial resource investment.
For patients, 3D assessment can provide more comprehensive information on embryo quality, helping clinicians select the most developmentally competent embryos for transfer, thereby improving pregnancy success rates and reducing the physical, psychological, and economic burdens associated with repeated transfers. Visual 3D models also enable patients to intuitively understand the status of their embryos, enhancing doctor-patient communication and treatment confidence. At the academic level, this study achieved full-process 3D reconstruction of blastocysts and large-scale quantification of 3D morphological parameters based on a large sample of 2025 blastocysts. It proposed novel predictive indicators such as ICM shape factor and ICM-TE spatial distance, providing important data support for research on embryonic development mechanisms.
Of course, the study also has certain limitations. The included blastocysts were all from FET cycles, mainly high-quality embryos, with a relative lack of low-quality blastocyst samples. The imaging range limitation of TL equipment may result in incomplete imaging of some blastocysts, affecting reconstruction accuracy. In the future, the research team will further optimize the technology, expand the sample size—especially by increasing research data on low-quality blastocysts—and explore combinations with other embryo assessment indicators, such as embryonic kinetic parameters, to further improve prediction accuracy.
With the continuous improvement of the technology, this AI-driven 3D blastocyst reconstruction technology is expected to become a standardized assessment tool in the field of ART, promoting embryo selection toward a more precise, objective, and automated direction. It is believed that in the near future, this technology will be widely applied in clinical practice, bringing hope for fertility to more infertile families and helping ART reach new heights.
Huang B, Si K, Guo Y, Wang X, Zhou W, Ma B, Ren X, Wu L, Yue J, Wang J, Shi Z, Jin L. Timelapse-based 3D reconstruction of blastocysts reveals 3D morphologies of human blastocysts. NPJ Digit Med. 2025 Nov 17;8(1):671. doi: 10.1038/s41746-025-02028-9. PMID: 41249434; PMCID: PMC12623974.