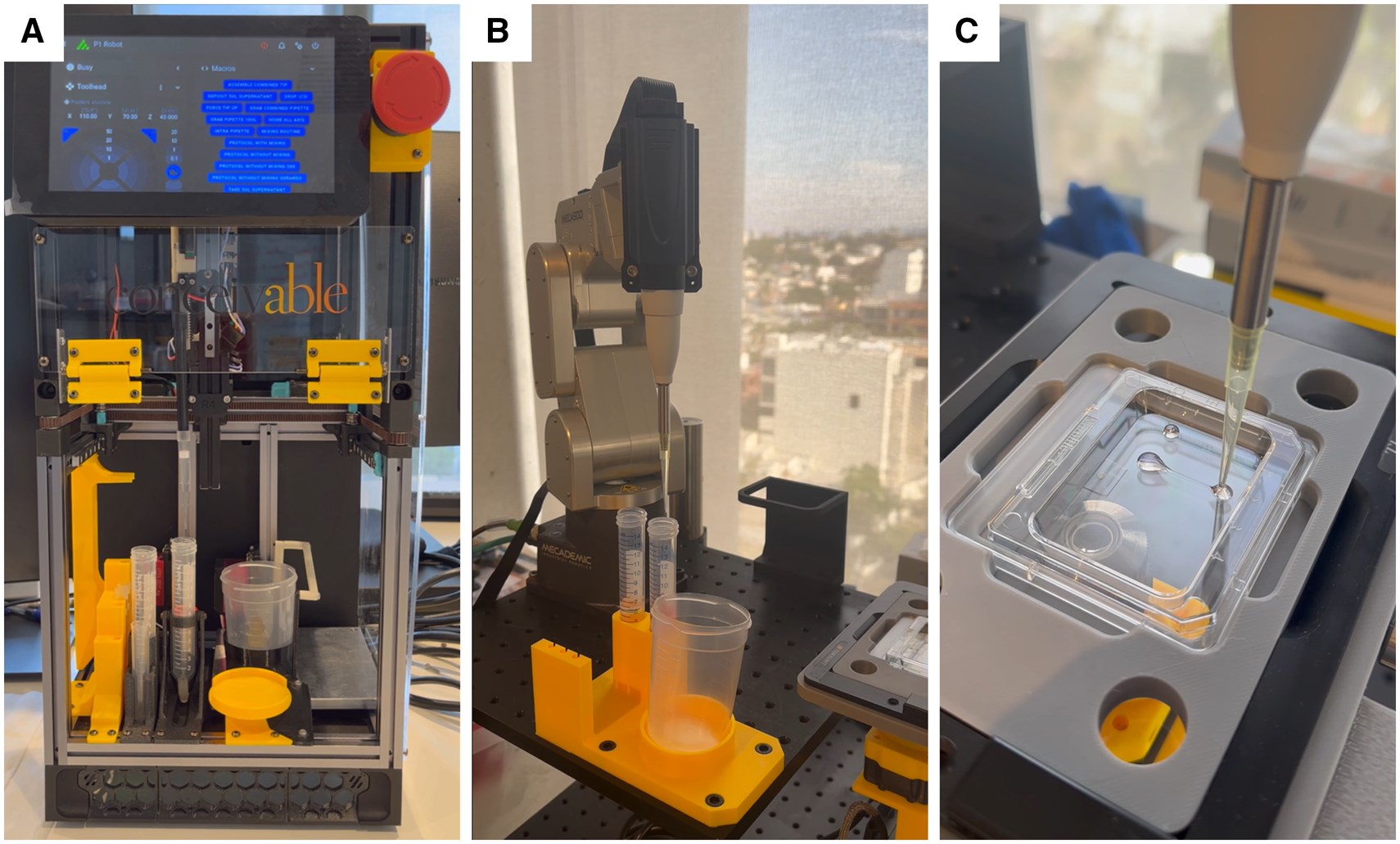
With the continuous growth in global demand for Assisted Reproductive Technology (ART), how to improve the standardization of laboratory operations and clinical efficiency through technical means has become a focus of the industry. Recently, a prospective proof-of-concept study published in Human Reproduction explored in detail the feasibility of using automated systems to sequentially execute the core processes of in vitro fertilization (IVF). Although the technology still has room for optimization in terms of automation level and success rate, its achievement of the first reported live birth marks a crucial step for IVF laboratories to move from the “manual era” to the “digital and automated era”.
Chavez-Badiola A, Mendizabal-Ruiz G, Flores-Saiffe Farías A, et al. Automated oocyte retrieval, denudation, sperm preparation, and ICSI in the IVF laboratory: a proof-of-concept study and report of the first live births. Hum Reprod. Published online December 26, 2025. doi:10.1093/humrep/deaf240

1. Research Background
The operations performed on the day of fertilization (Day 0) in traditional IVF laboratories, including sperm preparation, identification and denudation of cumulus-oocyte complexes (COCs), and intracytoplasmic sperm injection (ICSI), are highly dependent on the manual skills and professional experience of embryologists. However, the high-intensity repetitive work not only easily causes operator fatigue but also leads to individual technical variations, which in turn affect the consistency of experimental results. Therefore, the introduction of artificial intelligence (AI) and robotic technologies to reduce human variability is regarded as a key pathway to promote the standardized development of the assisted reproductive field.
2. System Architecture
The study deployed three independent automated units called “Pearls”, each of which was technically customized for different links on Day 0:
Pearl 1 (Sperm Preparation): This system integrates two automated pathways to handle semen samples of varying quality. Pearl 1 SU (Swim-up) achieves medium stratification in centrifuge tubes through robotic precision pipetting, simulating the traditional swim-up method; Pearl 1 HSO (Horizontal Swim-out) uses custom horizontal discs to allow sperm to migrate horizontally into the collection area. The core advantage of both lies in the efficient separation of highly motile sperm without physical centrifugation through precise fluid control, thereby minimizing the potential damage to sperm DNA structure caused by reactive oxygen species (ROS) generated during centrifugation.
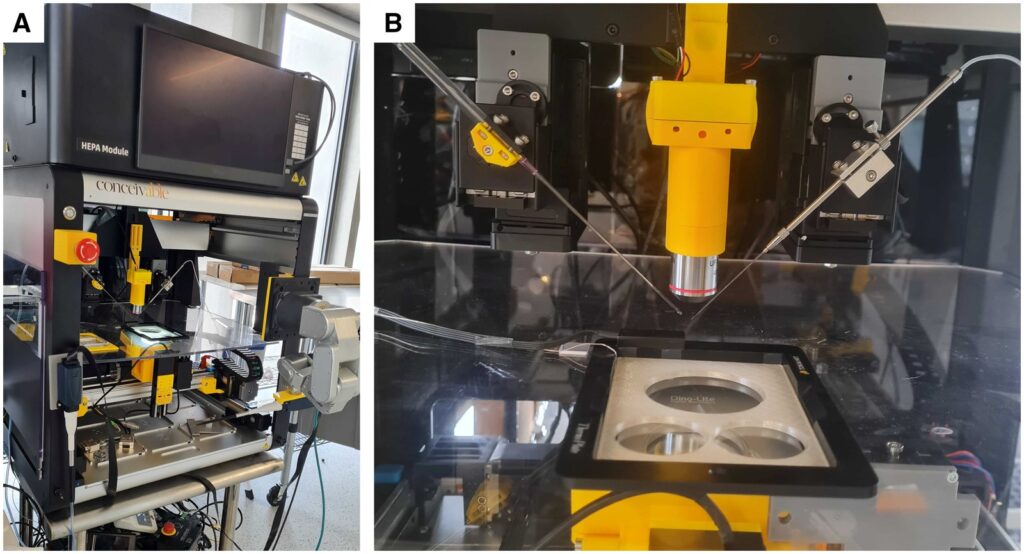
Pearl 2 (Oocyte Retrieval & Denudation): This unit is equipped with dual Cartesian robotic arms and a high-resolution computer vision system. The AI model can scan petri dishes containing follicular fluid in real time, accurately locate COCs within them, and guide the robot to aspirate them to the cleaning area. Subsequently, the system executes an automated denudation process, performing precise pipetting operations via a robot-controlled micropipette to remove cumulus cells surrounding the oocyte using hyaluronidase and mechanical shearing force. The process is digitally monitored, allowing operators to adjust pipetting intensity via video streams to ensure thorough denudation without damaging the oolemma.
Pearl 3 (ICSI): This is the most complex automated link in the entire process. The system uses an AI algorithm called “SiD” to screen sperm with optimal morphology and motility in microfluidic channels or dedicated dishes. Once selected, Pearl 3 immobilizes the sperm using laser pulses and performs injection with a high-precision robotic arm coupled with a piezo-actuator. Compared with traditional manual ICSI, this system significantly reduces the rate of oocyte deformation and damage during injection through preset puncture paths and submicron-level motion precision.
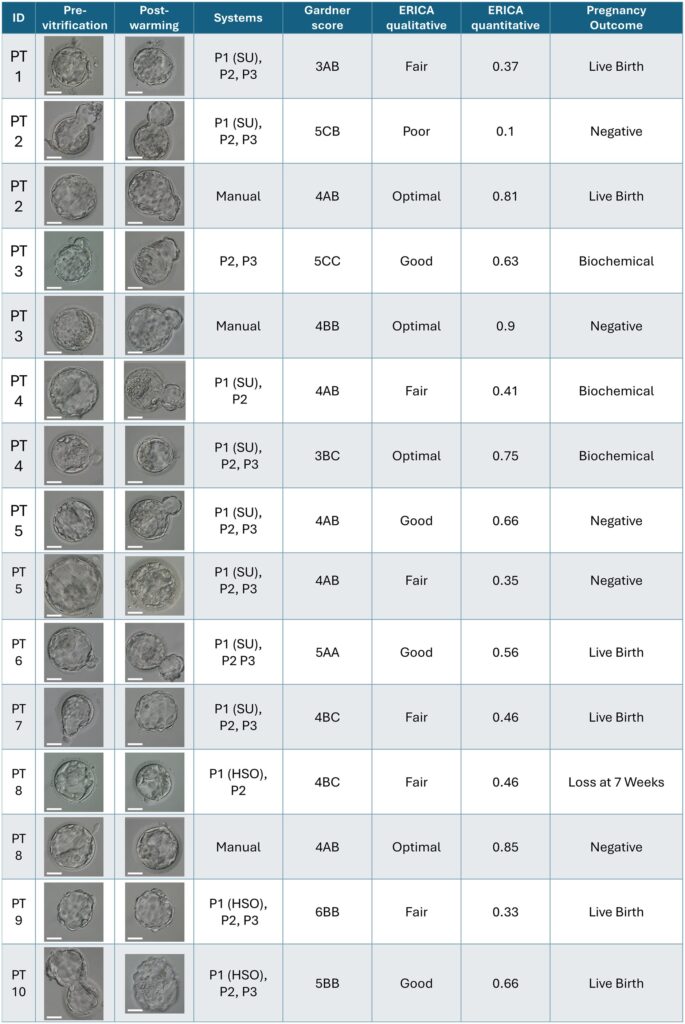
3. Study Design and Experimental Results
A total of 11 patients undergoing autologous or donor oocyte cycles were enrolled in this study. Oocytes were randomly assigned to the automated experimental group and the traditional manual control group using a sibling oocyte randomization method to ensure consistency in evaluation benchmarks. Experimental data showed that the automated system exhibited good technical feasibility in sequentially performing Day 0 operations: the automated group achieved a fertilization rate of 64.3% (45/70) and a usable blastocyst formation rate of 42.2%. Although numerically slightly lower than the manual control group (fertilization rate 81%) due to the preliminary debugging phase, the system achieved a significant breakthrough in clinical outcomes. Through single blastocyst transfer of embryos generated by the automated group, the research team successfully recorded 5 cases of healthy live births, with a live birth rate of 55.6% among patients with positive pregnancy tests and a live birth rate per transfer cycle of 41.7%.
4. Innovation and Limitations
The innovation of this study lies in its first clinical demonstration of the feasibility of sequentially executing the full Day 0 IVF process through multi-system integration, breaking the previous limitation of automation being confined to a single link and successfully translating digitally guided operations into the birth of healthy offspring. However, as a proof-of-concept study, its limitations are also evident. First, the system has not yet achieved fully autonomous operation; manual intervention is still required for sample transfer between devices, consumable replacement, and key decision-making points (such as final sperm confirmation). Second, due to the small sample size (n=11), the study cannot provide sufficient statistical evidence to prove that automation is superior to manual operation. Finally, the system currently has room for optimization, as its fertilization rate is still lower compared with top-level manual operations.
5. Conclusions and Future Outlook
This study initially established a clinical paradigm for automated Day 0 IVF processing, demonstrating that embryos generated through automated operations possess complete developmental potential and the ability to produce healthy offspring. The significance of this model lies in its ability to significantly reduce reliance on operators’ physical skills, providing a new technical framework for the standardization of laboratory operations. With the improvement of hardware integration and further training of AI algorithms, future R&D focus will be on enhancing the autonomy and scalability of the system to meet the increasingly severe global demand for fertility medical services.