As an authoritative standard in the field of Assisted Reproductive Technology (ART), the Istanbul Consensus on Embryo Assessment has systematically updated the embryo evaluation system by integrating technological breakthroughs over the past decade—such as Time-Lapse Technology (TLT) and Preimplantation Genetic Testing for Aneuploidy (PGT-A)—along with extensive clinical embryo data. This article focuses on the core changes in the evaluation criteria for key stages of embryo development, particularly supplementing indicators that were previously overlooked and explicitly identified in the Consensus as “insufficiently proven to be associated with embryo quality.” It aims to provide more comprehensive and precise references for embryologists and reproductive medicine practitioners.
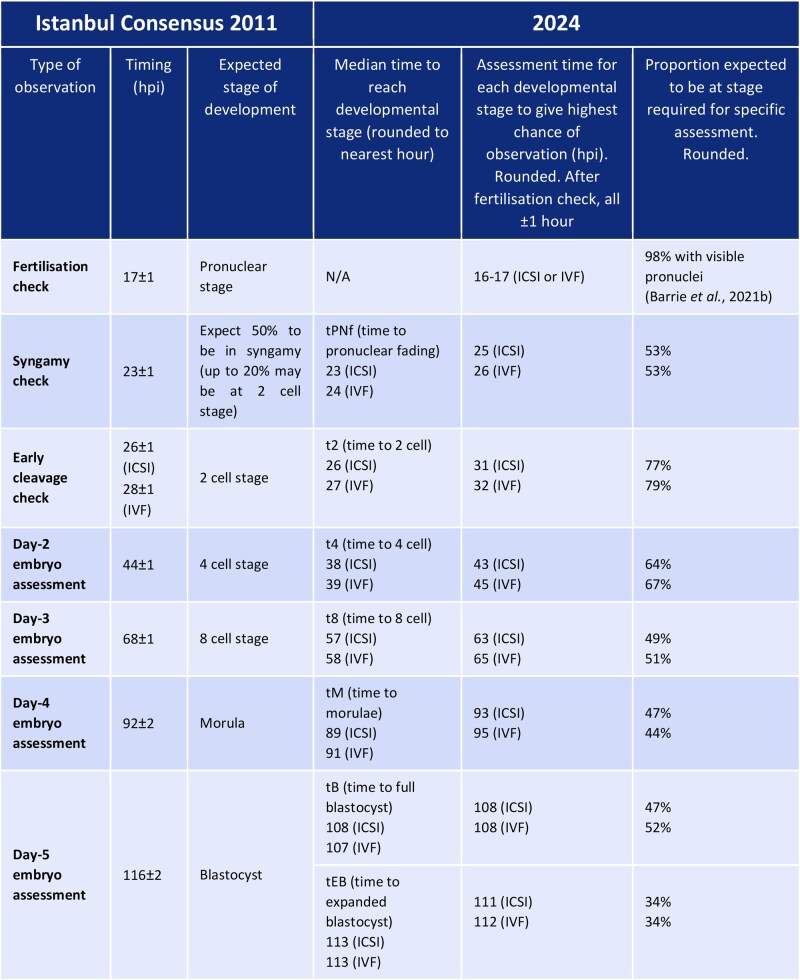
Embryo Development Timeline: “hpi” as the Benchmark, with Clear Influencing Factors
One of the most core adjustments in the new Consensus is the shift from the traditional “days post-insemination” (e.g., Day 1, Day 2) to hours post-insemination (hpi) as the unified benchmark for observing embryo development. This change completely resolves the challenge of “asynchronous development of embryos labeled as the same day” across different laboratories and under varying culture conditions, laying a critical foundation for the standardization of cross-center data. Based on extensive clinical research, the Consensus further clarifies three core factors influencing embryo development timing:
- Impact of fertilization method on early development: Only the timing of the first cleavage is affected by the fertilization method—embryos derived from ICSI reach the 2-cell stage (t2) 0.98 hours earlier than those from IVF (26 hpi vs. 27 hpi). However, the time of blastocyst formation initiation (tSB) and the time to fully formed blastocyst (tB) are 1.157 hours and 1.510 hours later, respectively. This discrepancy indicates that early cleavage speed cannot fully represent subsequent developmental potential; instead, a comprehensive assessment of the embryo’s full kinetic characteristics is required to avoid misjudging embryo viability based solely on a single time point. Current research shows that some ICSI embryos, despite rapid early cleavage, may experience developmental arrest during the critical stage of blastocyst formation due to issues such as cell rearrangement or gene expression regulation.
- Temporal characteristics of aneuploid embryos: Studies have shown that aneuploid embryos exhibit significant delays in 10 morphokinetic parameters from the 8-cell stage (t8) to the expanded blastocyst stage. In particular, the prolongation or acceleration of the cell cycle may be associated with abnormal DNA repair activity, impaired cell rearrangement, or failure of cell cycle checkpoints. This finding provides an important basis for the preliminary screening of euploid embryos using TLT, helping laboratories narrow down the screening range before genetic testing.
- Critical role of the culture environment: Environmental factors significantly affect embryo development speed: Embryos cultured in a 20% atmospheric oxygen concentration develop more slowly than those in a 5% physiological oxygen concentration, with a reduced implantation rate. A shift in medium pH toward alkalinity or a decrease in temperature slows embryo development; the type and composition of the medium also influence embryo morphokinetics, so differences in medium should be noted when comparing research results. Ovarian stimulation protocols may affect the development timing of embryos during the early cleavage stage but do not impact overall embryo quality. Laboratories must strictly control the stability of the culture environment, record the protocols used, and ensure data traceability.

Changes in Pronuclear Stage Assessment
The pronuclear stage is critical for determining fertilization effectiveness and directly affects the evaluation of subsequent embryo developmental potential. Based on new findings from TLT and PGT-A technologies, the new Consensus introduces important adjustments to assessment timing, pronuclear number terminology, and clinical selection. It also clarifies several indicators for which “current evidence is insufficient to confirm an association with embryo quality,” preventing misjudgment caused by overinterpretation:
1. Assessment Timing: 16–16.5 hpi as the Optimal Static Observation Window
The 2011 version of the Consensus recommended assessing pronuclei at 17 ± 1 hpi. However, the new Consensus, based on a large-scale TLT study involving numerous IVF and ICSI embryos, confirms that 16–16.5 hpi is the optimal time for static observation of pronuclear number. During this window, the visibility rate of 2 pronuclei (2PN)—indicating normal fertilization—is the highest (>98%), maximizing the detection of normally fertilized pronuclei. If assessment is conducted after 17 hpi, some pronuclei may undergo early pronuclear breakdown (PNBD) and be misjudged as “unfertilized,” leading to the erroneous discarding of viable embryos. Therefore, laboratories must strictly adhere to this pronuclear assessment time window to avoid timing-related inaccuracies.
2. Pronuclear Number: Updated Terminology and Clinical Value Judgment
- “Pronuclei not observed” replaces “0PN”: For cases where no pronuclei are observed during static observation but the embryo develops normally afterward, the Consensus explicitly abandons the term “0PN” (no pronuclei) and adopts “pronuclei not observed” instead. Overall morphokinetic evidence does not confirm that “embryos without pronuclear formation can still develop.” On the contrary, it is highly likely that “0PN” embryos that progress to the first mitosis are actually 2PN, or in rare cases 1PN/multiple pronuclei, which underwent PNBD before pronuclei could be detected during static fertilization assessment. Since PNBD occurs earlier than the traditional assessment time point, it cannot be captured during static observation—but the embryo itself still has normal developmental potential, with a live birth rate comparable to that of normal 2PN embryos. Such embryos should not be directly classified as “unfertilized.” In fact, embryos that exhibit faster morphokinetic characteristics during the fertilization stage generally have stronger developmental capabilities.
- 1PN pronuclei: In the past, 1PN embryos were often regarded as abnormal and discarded. However, PGT-A technology has rehabilitated their clinical value. Studies indicate that approximately 40%–50% of 1PN blastocysts are biparental diploids with a normal chromosome composition when tested by PGT-A. In terms of implantation rate, pregnancy rate, and live birth rate, 1PN blastocysts perform excellently, comparable to traditionally normally fertilized 2PN blastocysts. Exploring their formation mechanism: in IVF cycles, some 1PN embryos result from asynchronous development of male and female pronuclei or their premature fusion. Although these embryos appear as “single pronuclei,” they have intact chromosome sets and the potential to develop into healthy fetuses. However, 1PN embryos are not without risks: in ICSI cycles, the blastocyst formation rate of 1PN embryos (17.4%) is significantly lower than that in IVF cycles (33.7%), indicating that the fertilization method has a significant impact on the subsequent development of 1PN embryos.
- 2.1PN pronuclei: 2.1PN pronuclei—referring to zygotes with 2 normal pronuclei accompanied by 1 micronucleus—are extremely rare, accounting for less than 1% of embryos. Previously, due to technical limitations and their “abnormal” pronuclear composition, they were often directly discarded in clinical practice. However, with in-depth research, especially the accumulation of clinical cases in recent years, it has been confirmed that 2.1PN embryos can also develop into biparental diploid blastocysts and eventually result in the birth of healthy-appearing infants. Relevant studies show that the developmental potential of 2.1PN embryos is influenced by multiple factors: 2.1PN zygotes from older patients (≥38 years old) have stronger blastocyst formation ability than those from younger patients, and the difference in developmental potential compared with 2PN zygotes narrows. When determining whether 2.1PN embryos are worth culturing, the early embryo cleavage pattern and Day 3 embryo quality are key predictive indicators—embryos with a normal cleavage pattern and high-quality grading have a higher chance of blastocyst formation.
- 3PN pronuclei: Most 3PN embryos result from digynic/diandric fertilization and carry an extremely high risk of aneuploidy. Clinical application cases are extremely rare, so routine use is not recommended for the time being. However, preclinical research is encouraged to explore their potential value (e.g., whether rare euploid cases exist).
3. Morphological Characteristics: Clarifying Dynamic Indicators “Insufficiently Proven to Be Relevant”
Many morphological characteristics of the pronuclear stage show dynamic changes, and current evidence is insufficient to confirm a strong association with embryo quality. Overreliance on these indicators during clinical assessment should be avoided:
- Pronuclear size and position: The paternal pronucleus is usually larger than the maternal pronucleus, and the size difference gradually decreases as fertilization progresses. However, existing studies have conflicting conclusions on whether “pronuclear size difference affects outcomes,” so it cannot be confirmed as a reliable predictive indicator. Only “extremely rare eccentric positioning” (with an extremely low incidence) of pronuclei is associated with abnormal cleavage; occasional positional differences observed in routine static observation are insufficient as a basis for excluding embryos.
- Nucleolar precursor body (NPB) pattern: The process of NPBs from condensation and aggregation to dispersion is continuous and dynamic. There are differences in the aggregation kinetics of NPBs between paternal and maternal pronuclei, and even after aggregation is complete, NPBs may actively disperse a few hours before pronuclear breakdown. Current studies show that NPB patterns have no clear predictive value for implantation and live birth outcomes; only studies that complexly calculate their movement speed have found weak correlations, which is insufficient to qualify NPB patterns as clinical assessment indicators.
- Cytoplasmic halo: A cytoplasmic halo is a region with reduced cytoplasmic granularity in the pronuclear cortical area, visible in 82%–98% of pronuclei. It typically forms 2–4 hours after pronuclei appear and disappears 1 hour before pronuclear breakdown. Studies have confirmed that the absence of a cytoplasmic halo affects blastocyst formation efficiency but does not impact the implantation rate of blastocysts after transfer. Thus, it can be used for ranking embryos in Day 3 embryo transfers but cannot serve as a basis for excluding embryos; if embryos are cultured to the blastocyst stage, the presence or absence of a cytoplasmic halo has no significant impact on outcomes.
In summary, the updates to the embryo development timeline and pronuclear stage assessment in the new Consensus provide more precise standardized guidelines for clinical practice. The development timeline uses hpi as the benchmark, and it is recommended that each center establish a reasonable assessment time based on its own dataset. Adjustments to the pronuclear stage are adapted to the application of time-lapse incubator systems, reducing misjudgments. The interpretation of cleavage stage and blastocyst stage assessment key points will be covered in subsequent articles.