Following the previous interpretation of the time benchmarks for embryo assessment and the new criteria for the pronuclear stageInterpretation of the New Istanbul Consensus (Part 1): Embryo Assessment Time Benchmark + New Pronuclear Stage Rules, this article will focus on the cleavage stage (Days 1-3)—a critical phase of embryonic development—outlined in the new Istanbul Consensus. As the core stage during which early embryos proliferate from the 2-cell to the 8-cell stage, the morphological and kinetic characteristics of the cleavage stage directly reflect an embryo’s developmental potential. The new Consensus has optimized and adjusted assessments for this stage, refining criteria around three core parameters: blastomere number, degree of fragmentation, and multinucleation. Below is a comprehensive presentation of the core content regarding cleavage stage assessment.
Core Application Value of Time-Lapse Technology (TLT) in Cleavage Stage Assessment
In cleavage stage embryo assessment, time-lapse technology (TLT) is a precision assessment tool strongly recommended in the new Consensus. Its core advantage lies in continuous dynamic observation, which addresses the subjective errors and information gaps of static observation. Its specific applications span the assessment of the three core parameters (blastomere number, fragmentation, and multinucleation), with the following unified functions:
- Provide precise time benchmarks: Based on extensive TLT data, the consensus defines the median developmental time for embryos at each cell stage under different fertilization methods, offering an objective basis for judging optimal developmental speed.
- Capture dynamic change characteristics: It can fully record the emergence, absorption, or excretion of fragments, as well as the changes in multinucleation ratio of multinucleated embryos from the 2-cell to 4-cell stages, avoiding misjudgments from single static observations.
- Reduce assessment interference: It can clearly distinguish between large fragments and small blastomeres, accurately record cell division timing and the incidence of abnormal division, and minimize judgment errors caused by variations in culture conditions and assessment timing.
- Improve abnormal detection rate: Compared with the 6%-7% multinucleation rate recorded by static observation, TLT assessment can reach 23%-35%, which is closer to the actual incidence and reduces missed diagnoses.
Therefore, the consensus suggests that laboratories with the capability should prioritize TLT for cleavage stage assessment, providing more comprehensive dynamic data support for embryo quality evaluation.

Blastomere Number: Optimal Developmental Speed as the Core Judgment Criterion
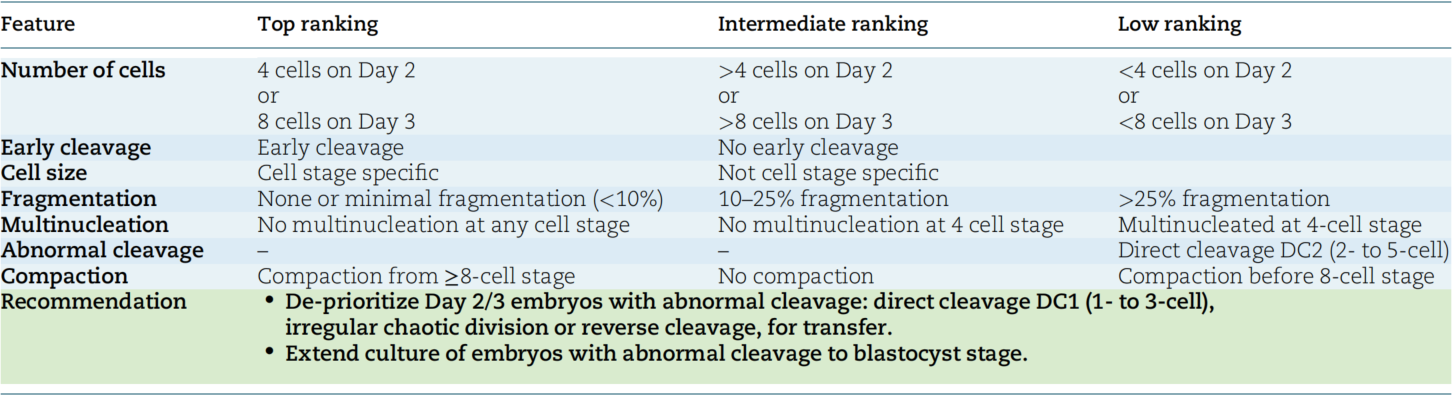
The consensus clarifies that the “optimal developmental speed” of cleavage stage embryos requires distinguishing specific time points based on fertilization methods. Additionally, there are differences between the timing of static observations and the actual time when embryos reach the corresponding cell stage, which must be defined separately:
- 2-cell stage (t2): The time for ICSI-derived embryos to reach the 2-cell stage is 26 hours post-insemination (hpi), while for IVF-derived embryos it is 27 hpi. This median time, derived from extensive TLT data, serves as a core reference for judging early cleavage speed.
- 4-cell stage: Static observation is recommended at 44±1 hpi (i.e., Day 2 post-insemination). At this point, the ideal developmental state of the embryo is 4-cell, but the actual time to reach the 4-cell stage varies by fertilization method—38-43 hpi for ICSI embryos and 39-45 hpi for IVF embryos. Approximately 64% (ICSI) to 67% (IVF) of embryos reach the 4-cell stage at this phase.
- 8-cell stage: Static observation is recommended at 68±1 hpi (i.e., Day 3 post-insemination). Here, the ideal developmental state is 8-cell, while the actual time to reach the 8-cell stage is 57-63 hpi for ICSI embryos and 58-65 hpi for IVF embryos. Around 49% (ICSI) to 51% (IVF) of embryos reach the 8-cell stage at this phase.
Embryos within the above speed ranges have significantly higher live birth rates than those outside the ranges. Clinical assessment should focus on alerting to two types of abnormal developmental conditions:
- Excessive development: When Day 3 embryos have more than 8 cells (e.g., 9-10 cells), the aneuploidy rate increases significantly, and the incidence of abnormal division patterns (such as tripartite division or direct division) is also higher. The blastocyst formation rate is lower than that of standard 8-cell embryos. However, if such embryos successfully develop to the blastocyst stage, their subsequent implantation potential is similar to that of 8-cell embryos. Therefore, direct exclusion at the cleavage stage is not recommended; instead, extended culture to the blastocyst stage for further assessment is advised.
- Slow development: Embryos with fewer than 4 cells on Day 2 and fewer than 8 cells on Day 3 consistently have poor outcomes. Studies have shown that the fertilization rate, blastocyst formation rate, and live birth rate of such embryos are significantly lower than those of normally developing embryos. They should only be considered for transfer when no better-quality embryos are available.
Fragmentation: Grading by Blastomere-Equivalent Volume and Clarifying Clinical Significance
Fragmentation is a common morphological feature of cleavage stage embryos, defined as membrane-enclosed extracellular cytoplasmic clumps that typically do not contain chromosomes but can affect embryonic viability. The new Consensus classifies fragmentation degree into three grades based on blastomere-equivalent volume (i.e., the ratio of fragment volume to normal blastomere volume) and specifies clinical management recommendations for each grade:
- No fragmentation/minor fragmentation (<10%): The preferred grade, with negligible impact on implantation rate. For example, if the fragment volume in a 4-cell embryo is less than 10% of the volume of a normal blastomere, the embryo’s developmental potential is essentially unaffected, making it the first choice for transfer or cryopreservation.
- Mild fragmentation (10%-25%): Suitable for clinical use, but requires comprehensive judgment combined with other parameters. Although such embryos have some fragmentation, most still have the potential to develop to the blastocyst stage. Continued culture and observation are recommended; if fragmentation does not further increase, their clinical application value can be retained.
- Severe fragmentation (>25%): Extended culture to the blastocyst stage is recommended for further assessment of developmental potential. Studies have shown that for each increase in fragmentation grade, the probability of live birth decreases significantly by 46%. Additionally, fragmentation is positively correlated with the incidence of aneuploidy—the more fragments, the higher the risk of chromosomal abnormalities. Therefore, severely fragmented embryos are not recommended for direct transfer at the cleavage stage; instead, blastocyst culture should be used to select embryos with stronger viability.
Multinucleation: Distinguishing Types and Stages to Avoid One-Size-Fits-All Judgments
Multinucleation is a common abnormality in cleavage stage embryos, but the impact of different types of multinucleation on embryonic potential varies significantly. The new Consensus explicitly requires distinguishing between binucleation and true multinucleation, and making comprehensive judgments based on the stage of occurrence:
- True multinucleation (≥3 nuclei per cell): Closely associated with reduced embryo implantation rates and increased aneuploidy risk. Studies have shown that the blastocyst formation rate of such embryos is lower than that of mononuclear embryos; even if they develop to the blastocyst stage, the aneuploidy rate is significantly higher. Caution is required in clinical application, and priority should be given to mononuclear embryos.
- Binucleation (2 nuclei in 2-cell stage): Unlike true multinucleation, TLT studies have shown that embryos with binucleation at the 2-cell stage have higher blastocyst formation and implantation rates than those with true multinucleation—even higher than some non-multinucleated embryos. Moreover, multinucleation exhibits dynamic changes: the proportion of multinucleation in embryos with multinucleation at the 2-cell stage decreases significantly when developing to the 4-cell stage (from 29%-43% to approximately 15%). Therefore, direct exclusion at the 2-cell stage is not recommended; continuous observation until the 4-cell stage is necessary for judgment.
Morphological Features with Insufficient Evidence for Association
Current evidence is insufficient to confirm that the following morphological features have a negative impact on cleavage stage embryo quality. These features should not be used as exclusion criteria in clinical assessment, and extended culture is recommended for further observation:
- Blastomere cytoplasmic characteristics: Including cytoplasmic vacuoles and central granulation. Some studies suggest that cytoplasmic vacuoles (especially large vacuoles with a diameter >25 μm) may disrupt the cytoskeleton, but the consensus notes that the presence of vacuoles is only associated with reduced fertilization rates, and evidence of negative effects on subsequent embryonic development is insufficient. Additionally, in ICSI procedures, the impact can be reduced by avoiding injecting sperm into vacuoles. Although some studies report that central cytoplasmic granulation is associated with decreased embryo quality and increased aneuploidy rate, analyses indicate that it may be a normal morphological feature of oocytes and is not sufficient as an exclusion criterion.
- Disordered spatial arrangement: Refers to blastomeres not arranged in the expected three-dimensional tetrahedral pattern (e.g., planar arrangement or excessive intercellular spaces). Studies have confirmed that the blastocyst formation rate and implantation rate of such embryos are not significantly different from those with normal arrangement. There is no conclusive evidence that they are abnormal embryos, so they can still be used clinically and do not need to be directly excluded due to arrangement issues.
- Zona pellucida abnormalities: Including zona pellucida thickening, surface irregularities, and increased density. Some studies suggest that zona pellucida abnormalities may reduce fertilization rates, but more studies show no association with embryo quality, implantation rate, or cryopreservation survival rate. Current evidence is insufficient to confirm negative prognostic value for embryonic potential, so cleavage stage embryos with different zona pellucida phenotypes are considered suitable for clinical use.
In the next interpretation, we will focus on the key points of blastocyst stage assessment and continue to explain the changes in the new Istanbul Consensus. Stay tuned.