Assisted Reproductive Technology (ART), a crucial means of addressing the global issue of infertility, has been expanding in scale but faces significant technological bottlenecks. Embryo pregnancy success rates have long stagnated around 30%, and overcoming age-related fertility decline remains a persistent challenge. Recently, a forward-looking review published in Nature Nanotechnology systematically outlines how the integrated innovation of artificial intelligence, micro-robotics, and nanobiosensors is poised to reshape the future technological landscape of reproductive medicine across three dimensions: automation of operations, precision in assessment, and minimally invasive diagnosis and treatment.
Striggow F, Jha P, Arora R, Medina-Sánchez M. Navigating the future of assisted reproductive technology with micro-robotics, nanobiosensors and artificial intelligence.Nat Nanotechnol. Published online December 29, 2025. doi:10.1038/s41565-025-02093-x

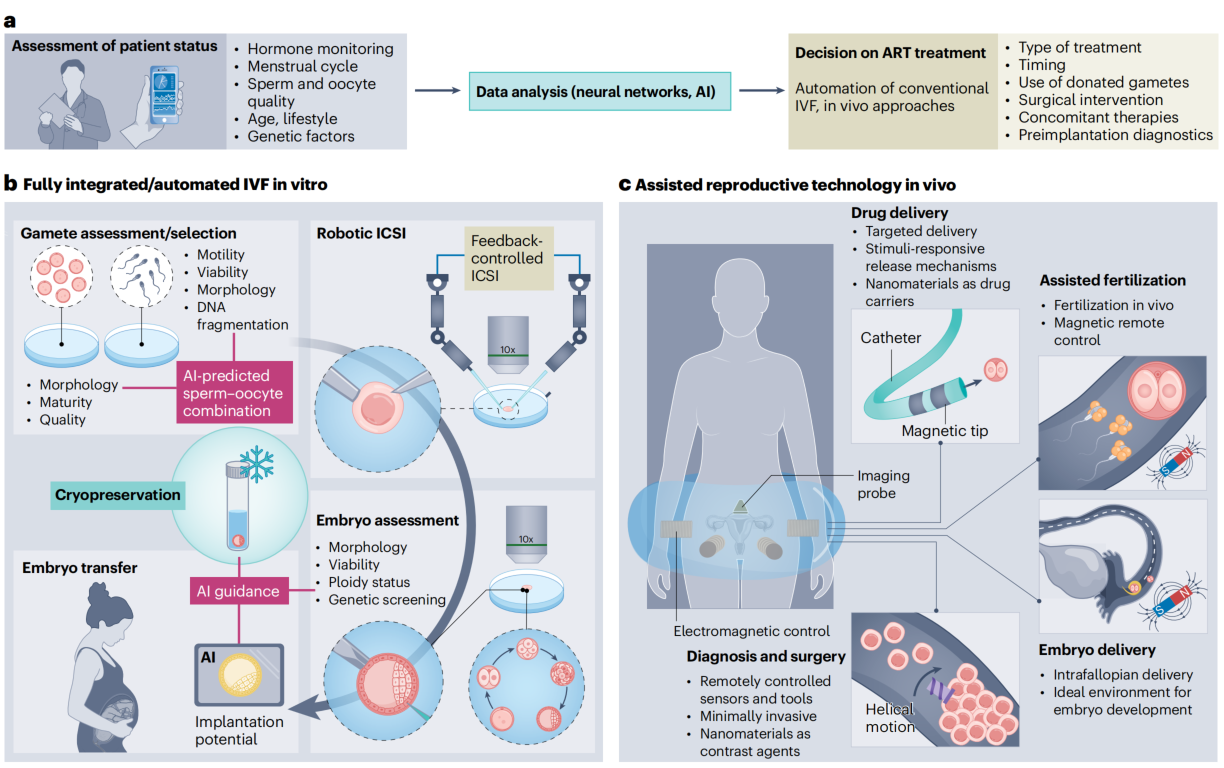
Automation of In Vitro Operations
Currently, the detection, selection, and manipulation of gametes and embryos primarily rely on manual techniques, where subjectivity and operational variability create bottlenecks for standardization and efficiency improvement. Computer-assisted sperm analysis (CASA) technology has pioneered the automated assessment of parameters like sperm motility and concentration, marking the starting point for ART automation. Building on this, deep learning and robotics are driving breakthroughs in more complex procedures: vision systems based on deep learning can precisely identify the polar body of oocytes and guide robotic micropipettes to perform oriented rotation, laying the foundation for automated Intracytoplasmic Sperm Injection (ICSI) and having undergone preliminary clinical validation. Automated three-dimensional reconstruction imaging and biopsy techniques for blastocysts further reduce human error. More innovatively, magnetic-mediated manipulation technology, utilizing magnetic nanoparticles modified with oviductal glycoprotein 1 (OVGP1), enables non-contact, precise manipulation of oocytes and embryos without affecting fertilization or offspring health. The successful development of dedicated magnetic micro-carriers also provides stable support for the contactless transport of gametes in vitro and the optimization of processing workflows.
Precision in Analysis and Assessment
Accurately selecting gametes and embryos with high developmental potential is a core element in improving ART success rates. Novel imaging and sensing technologies are now providing unprecedented technical support. Microfluidic systems, whether passive in design or active forms combined with electric or acoustic fields, have been applied to select sperm cells with lower DNA fragmentation indices, enhancing sperm quality through unique physical sorting mechanisms. Meanwhile, hyperspectral imaging technology demonstrates significant potential by enabling direct, non-invasive detection of various metabolites within oocytes and embryos, thereby quantifying their metabolic activity. When combined with AI algorithms, this technology holds promise for the objective classification of oocytes and embryos based on developmental potential.
At the microscopic scale, quantum sensing technology—methods of measurement based on phenomena at the atomic level—is pushing sensor precision to new heights. For instance, using diamond magnetometers in localized nanoscale magnetic resonance imaging, this technology has been employed to quantify free radicals within the sperm acrosome, opening a new microscopic dimension for sperm quality assessment. Addressing the long-standing technical challenge of non-invasive genetic screening of embryos, researchers have made significant progress: an AI-assisted nanosensor has been successfully developed. It can perform screening by analyzing trace genetic health biomarkers present in the embryo culture medium, thereby avoiding the potential impact of traditional invasive biopsies on the embryo.
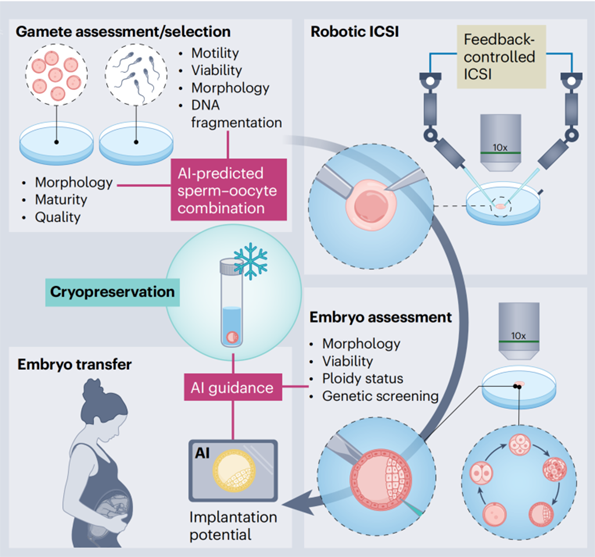
Minimally Invasive Diagnostic and Therapeutic Intervention
Wirelessly controlled micro- and nanorobots offer revolutionary prospects for performing delicate, minimally invasive operations on sensitive reproductive cells. Among these, Magnetic Micro/Nanorobots (MNRs), which integrate magnetic materials and are driven by external magnetic fields, can precisely control the rotation and translation of oocytes or embryos, optimizing in vitro observation and handling processes. For example, MNRs driven by rotating magnetic fields have achieved the remote-controlled transport of sperm cells. Dedicated magnetic micro-carriers can also be used for the contactless transport of oocytes and sperm cells in vitro, optimizing the process of gamete encounter through precise manipulation. Their size, comparable to that of gametes, lays the groundwork for future minimally invasive operations within narrow physiological channels like the fallopian tubes.
Beyond optimizing in vitro procedures, the core potential of MNRs lies in their expansion into in vivo minimally invasive diagnostic and therapeutic scenarios. They can act as intelligent carriers to deliver sperm targeted to the fertilization site in the fallopian tube or to precisely place an embryo at the optimal implantation point in the uterus, thereby reducing the invasiveness of traditional transfer operations. Furthermore, serving as platforms for drug or gene delivery, MNRs can enhance the targeting of treatments for gynecological diseases like ovarian cancer, minimizing side effects associated with systemic medication. By integrating multiple functional modules such as hyperthermia and imaging, their application scenarios will further expand.
Interdisciplinary Integration and Future Outlook
Advances in artificial intelligence, materials science, and micro/nano-fabrication are giving rise to novel technological tools in the field of assisted reproduction. Although challenges remain for the in vivo application of Magnetic Micro/Nanorobots (MNRs), their value in in vitro automated operations (such as ICSI and embryo manipulation) is already evident. Combined with AI-powered quality assessment, they hold promise for efficiently selecting high-quality gametes and embryos. In the long term, related technologies could extend to areas like contraception and gynecological disease treatment. Currently, AI and in vitro gametogenesis (IVG) are advancing rapidly. AI can non-invasively predict embryo ploidy, while IVG technology can generate gametes from somatic cells, offering hope for specific patient groups. However, the clinical translation of stem cell-derived human embryos needs to overcome significant technical and ethical hurdles. Stem cell therapies are also under exploration and may expand the pathways of assisted reproduction.
The complexity of the in vivo reproductive environment is a major obstacle to the clinical translation of novel micro/nano technologies. The fusion of micro/nano technology with tissue engineering and materials science can support research at the cellular and molecular levels of reproductive processes, bridging the gap between basic research and clinical application. Recent studies constructing embryo microenvironment models have clarified the critical role of mechanical forces in implantation. Future integration with nanotechnology could further assess embryo implantation potential. Additionally, nanobiosensors can monitor reproduction-related indicators, and nanomaterials can aid in constructing biomimetic reproductive microenvironments. These tools will deepen the understanding of reproductive biology and drive the development of safer and more efficient infertility treatment protocols.