In the era of the rapid development of Assisted Reproductive Technology (ART), how to accurately and non-invasively evaluate the quality of oocytes and embryos has long been a core challenge in both clinical and scientific research fields. The metabolic activity of oocytes and embryos is directly correlated with their developmental potential; metabolic abnormalities may lead to cell death, developmental arrest, or implantation failure. Traditional evaluation methods either rely on invasive sampling or only allow static morphological observation, making it difficult to reflect the real physiological state. Recently, a study published in Human Reproduction proposed a label-free, low-energy time-lapse metabolic imaging technology, offering an entirely new approach to address this long-standing problem.
Horta F, Vuyyuru A, Newman H, et al. Investigating metabolic activity during oocyte and early embryo development through label-free metabolic imaging: a systematic approach for timelapse applications. Hum Reprod. 2025;40(12):2272-2285. doi:10.1093/humrep/deaf196
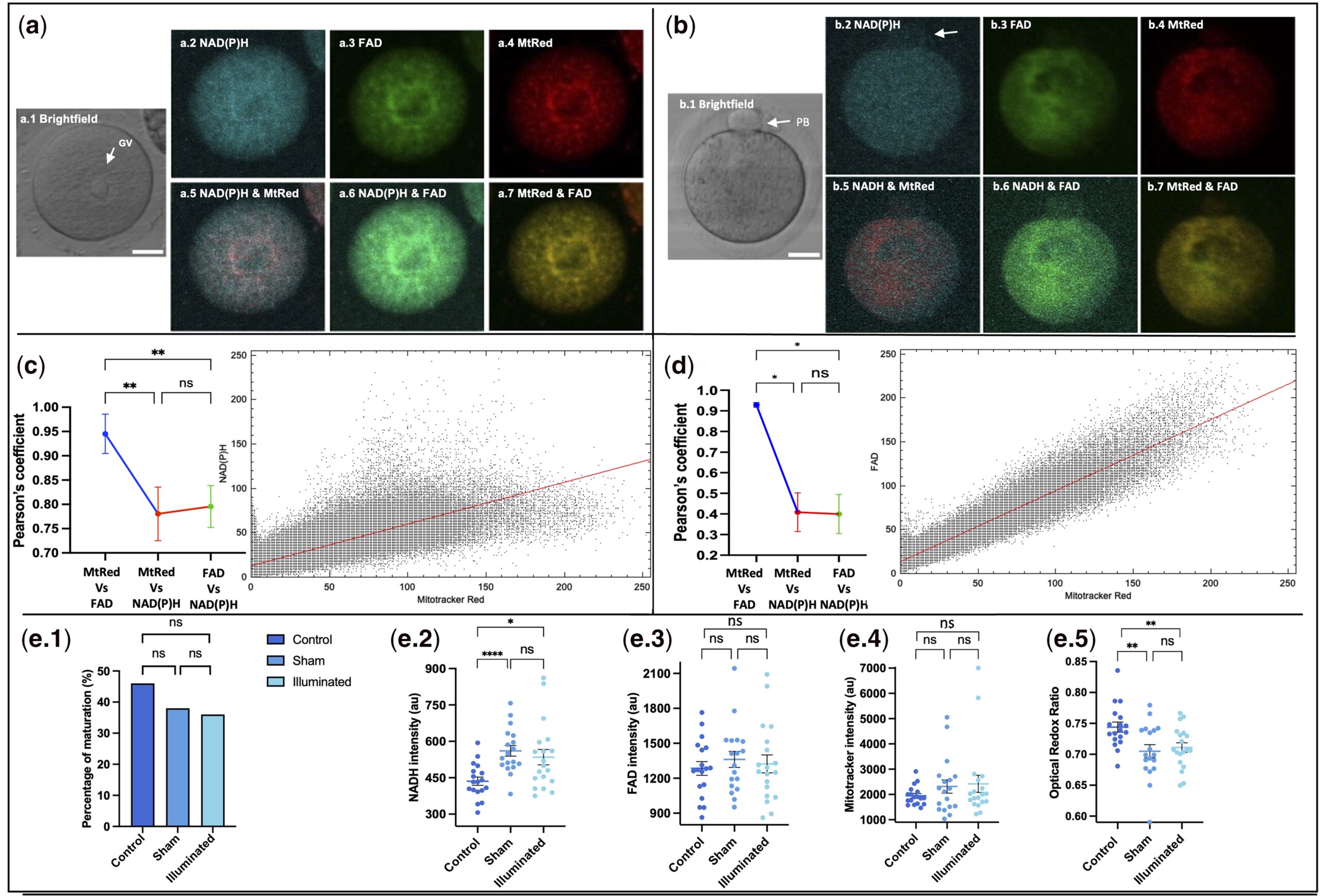
NAD(P)H and FAD autofluorescence from immature oocytes show high colocalization with Mitotracker Red fluorescence.
Core Technology
The core innovation of this technology lies in the in-depth integration of label-free detection and time-lapse monitoring. In terms of technical principles, it cleverly leverages the intrinsic fluorescence properties of two key intracellular metabolic molecules—NAD(P)H and FAD. These two molecules directly reflect mitochondrial function; the optical redox ratio (ORR), derived from their fluorescence intensity and the ratio between them, can accurately characterize cellular metabolic activity. By eliminating the need for any chemical labels, this technology fundamentally avoids potential damage to oocytes and embryos.
In terms of experimental operation, the research team established precise technical parameter standards. The imaging equipment adopted a confocal microscope, which used 405 nm and 488 nm lasers to excite fluorescence respectively. To ensure cellular safety, the laser energy was strictly controlled below 50 J·cm⁻², a level far lower than the damage threshold.
For monitoring frequency, imaging was performed every 3 hours to continuously track the entire developmental process of oocytes (18 hours) and embryos (66 hours). Meanwhile, Z-stack technology was used to record 15 focal planes, ensuring the completeness of imaging. In addition, the study combined immunostaining, in vitro culture and live birth experiments to form a complete safety verification chain.
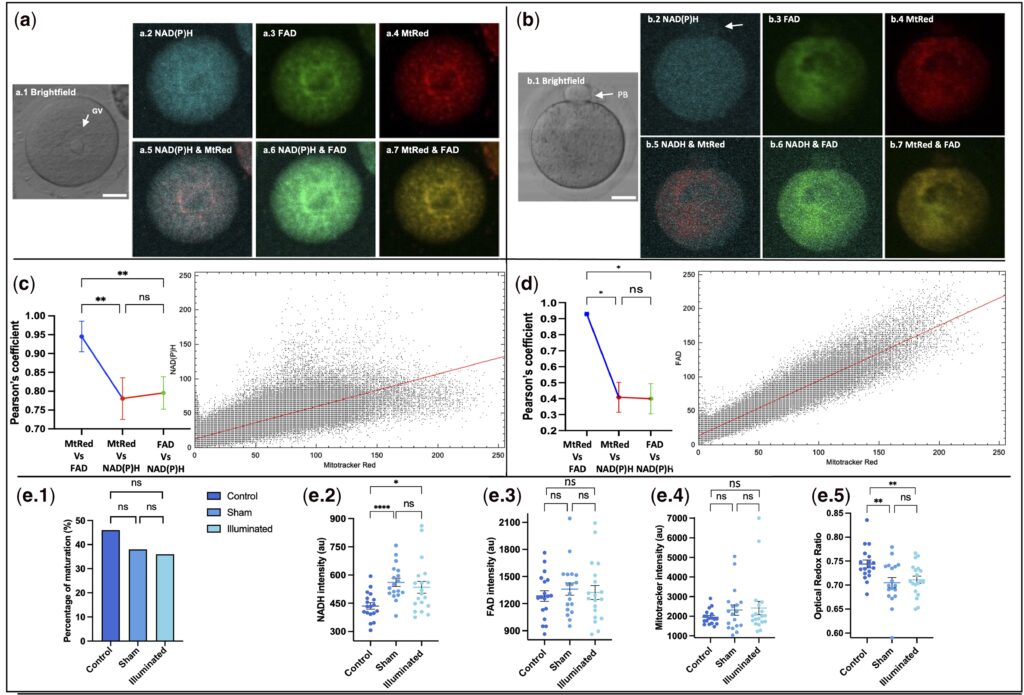
Changes in metabolic activity during oocyte IVM measured by confocal microscopy.
Experimental Design
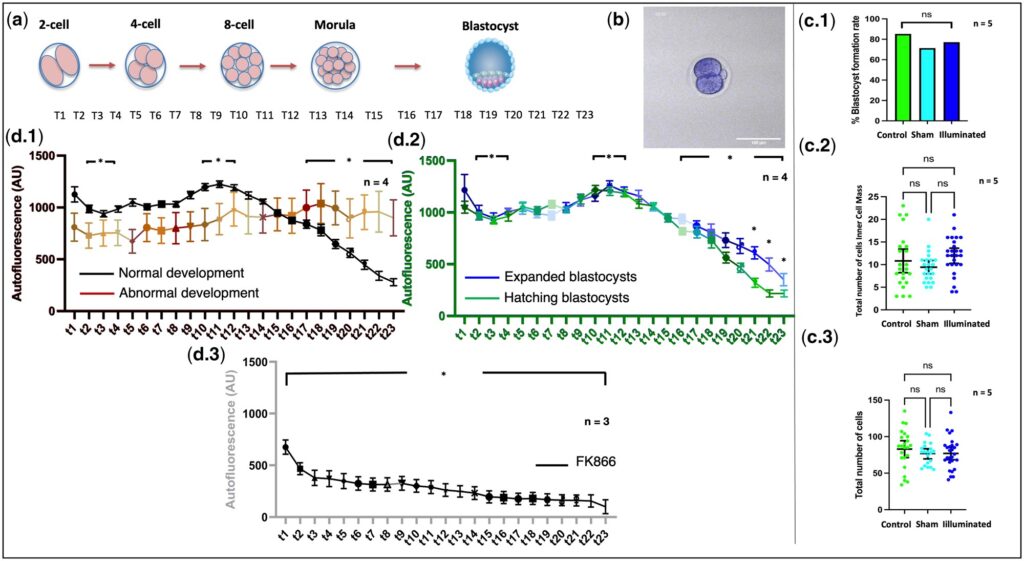
The experimental design of the study followed the principle of rigor throughout. From group control settings to the detection system, every step provided solid support for the reliability of the conclusions. In terms of group setup, the control group served as a standard reference to reflect the normal developmental status of oocytes and embryos; the sham control group excluded the interference of irrelevant factors such as microscope operation, ensuring that light exposure was the only variable; the light-exposed group underwent treatment with the core technology. The three groups formed a distinct comparison, clearly demonstrating the actual impact of the technology on cell development. The detection system covered three dimensions: metabolic signals, developmental indicators, and safety endpoints.
At the metabolic signal level, metabolic dynamics were captured by measuring fluorescence intensity and ORR values; at the developmental indicator level, key parameters including oocyte maturation rate, embryo blastocyst formation rate, total number of blastocyst cells, and number of inner cell mass (ICM) cells were monitored; at the safety endpoint level, the biosafety of the technology was comprehensively evaluated using indicators such as in vitro embryo growth area, live birth rate, offspring body weight, and health status.
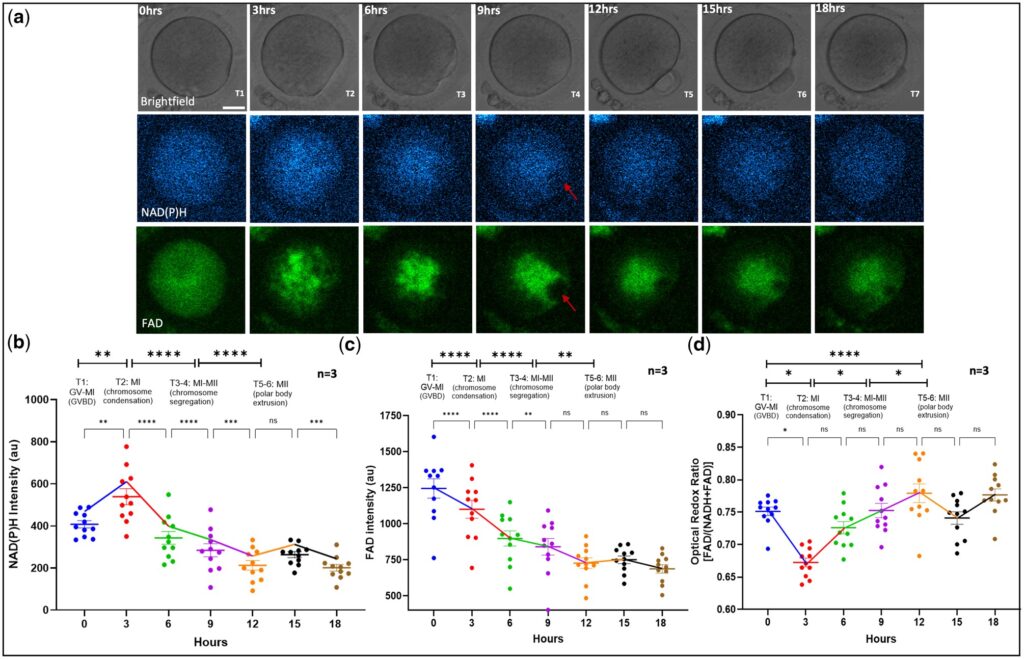
NAD(P)H intensity levels during early embryo development.
Core Findings
Systematic tests conducted in the study clearly demonstrated that there were no significant differences between the light-exposed group and the control group in key indicators, including oocyte maturation rate, blastocyst formation rate, and the postnatal health status of offspring. This fully verified the excellent biosafety of the technology.
In terms of metabolic dynamic patterns, the study found that the optical redox ratio (ORR) increased continuously during oocyte maturation, indicating that metabolic activity was enhanced along with the maturation process. More clinically meaningful is that the NAD(P)H levels during embryo development exhibited characteristic dynamic changes: in normally developing embryos, NAD(P)H levels rose gradually at the two-cell stage, peaked at the morula stage, and then decreased significantly at the blastocyst stage; in contrast, embryos that failed to develop into blastocysts showed significantly lower NAD(P)H signals at both the two-cell and morula stages. This finding indicates that the fluorescence signals of metabolic molecules can serve as biomarkers for predicting embryo developmental potential, providing a brand-new approach for the precise selection of high-quality embryos.
Innovative Value
The innovative highlights of this technology are concentrated in its achievement of the dual goals of dynamic monitoring and non-invasiveness. It breaks the limitations of previous metabolic imaging methods that relied on single-point detection or chemical labeling, making the assessment more consistent with the physiological state. In terms of indicator correlation, the study clarified the strong correlation between metabolic signals and developmental potential, confirming that the dynamic changes of endogenous molecules can effectively predict embryo developmental outcomes, thus changing the traditional assessment model that solely depended on morphology. Meanwhile, the research established a set of safe and controllable technical standards covering laser wavelength, energy dosage, and imaging frequency, laying a solid methodological foundation for the clinical translation of this technology.
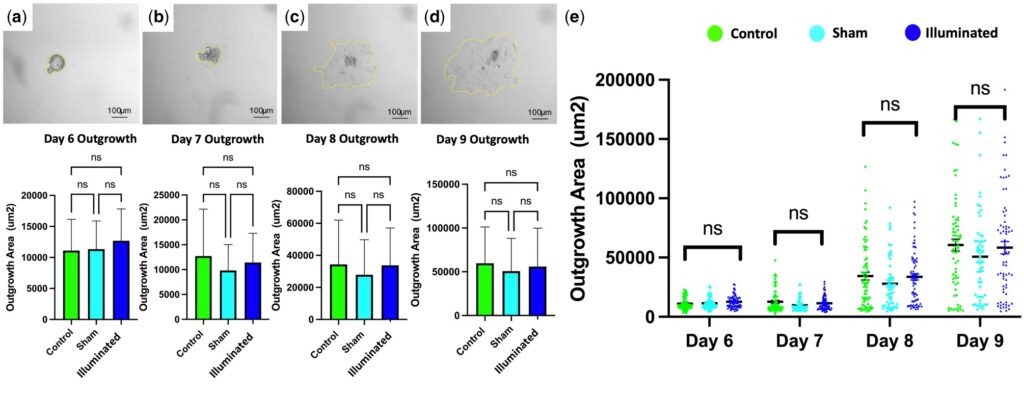
Embryo outgrowth by area assessment between study groups.
Research Limitations
Despite its great potential, the study objectively points out that there is a gap between animal models and clinical applications, and future validation needs to be carried out in human germ cells. In terms of technical parameters, the current excitation wavelength has not yet reached the optimal peak and the image resolution still has room for optimization, which needs to be further improved on the premise of ensuring safety. In terms of safety assessment, in-depth detection of long-term developmental risks such as DNA damage or gene mutations has not been involved yet. In addition, the experimental design can be further refined in the future to analyze the impact on cumulus cells, and the in vitro maturation rate of oocytes can be improved by optimizing the culture system, thereby making the evaluation indicators more comprehensive.
Conclusion
The advent of label-free time-lapse metabolic imaging technology has provided a brand-new paradigm for quality assessment in the field of assisted reproduction. With its core advantages of non-invasiveness, dynamic monitoring and safety, this technology breaks through the limitations of traditional evaluation methods, and can be seamlessly integrated with clinical time-lapse incubators to achieve real-time full-process monitoring of embryo development. It provides key support for the accurate prediction of embryo developmental potential and the selection of high-quality oocytes and embryos, and is expected to become a core tool for in vitro fertilization (IVF) technology in the future.