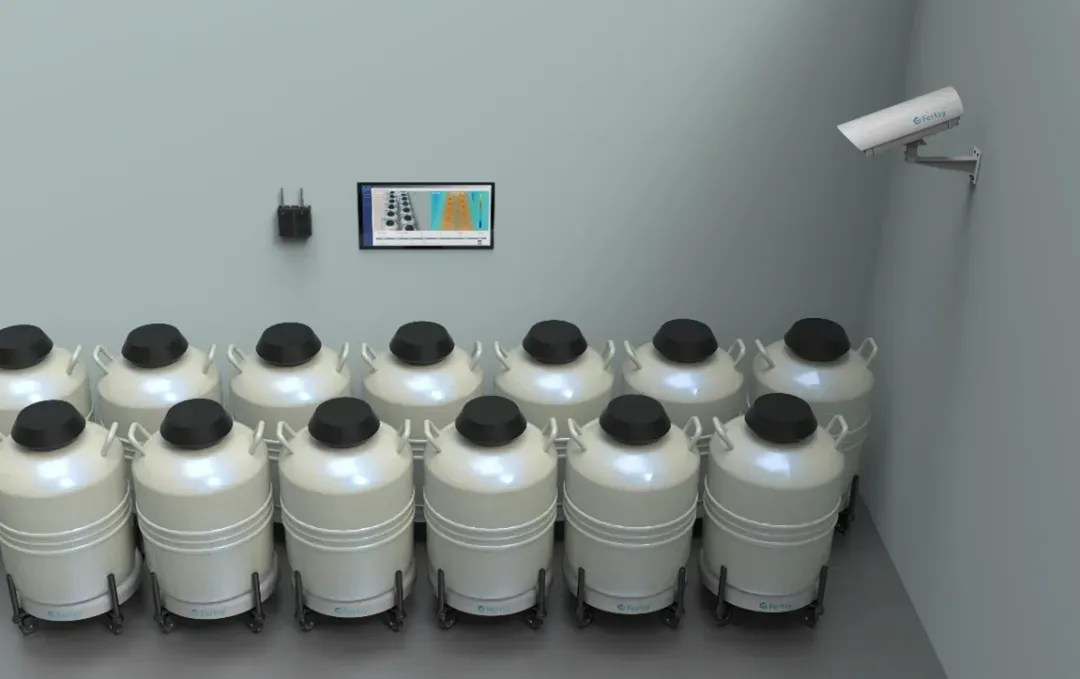
Fertsy (Beijing) Technology Co., Ltd., aiming to address the growing pressure of liquid nitrogen storage management , has designed and developed the brand-new three-in-one AI monitoring system, CryoTrio™, based on the existing equipment system and management processes of reproductive centers.
The CryoTrio™ AI monitoring system has successfully obtained EU CE certification and US FCC certification, marking that the system meets international standards in terms of safety and reliability. CE certification is a mandatory safety certification implemented by the EU for products entering its market, while FCC certification is a market access certificate for the US, focusing on the safety certification of wireless transmission equipment. Products failing to obtain FCC certification are prohibited from being sold in the US. The system adopts wireless data transmission, eliminating cable constraints and minimizing the impact on daily laboratory operations. These certifications not only prove that the system meets the access requirements of European and American markets but also reflect its high reliability and safety in medical environment applications.


Currently, the CryoTrio™ intelligent liquid nitrogen storage monitoring system has been deployed and put into use at IVF Canada’s Fertility Clinic Toronto and Aspire Houston Fertility Institute in Houston. The system is operating stably, and these clinical practice cases have verified its applicability in the international high-end medical market.


The successful acquisition of CE/FCC international certifications by the CryoTrio™ liquid nitrogen storage monitoring system signifies that the product has reached an internationally leading level in terms of safety and reliability. This important milestone has laid a solid foundation for the system to enter the high-end medical markets in Europe and America. In the future, our company will continue to safeguard the safety of every embryo with innovative technologies, and protect the birth of new lives.